- Home Page
- About
- Products
- News&Article
- Services
- Learning
- Contact
-
Order Product Form

Product Details
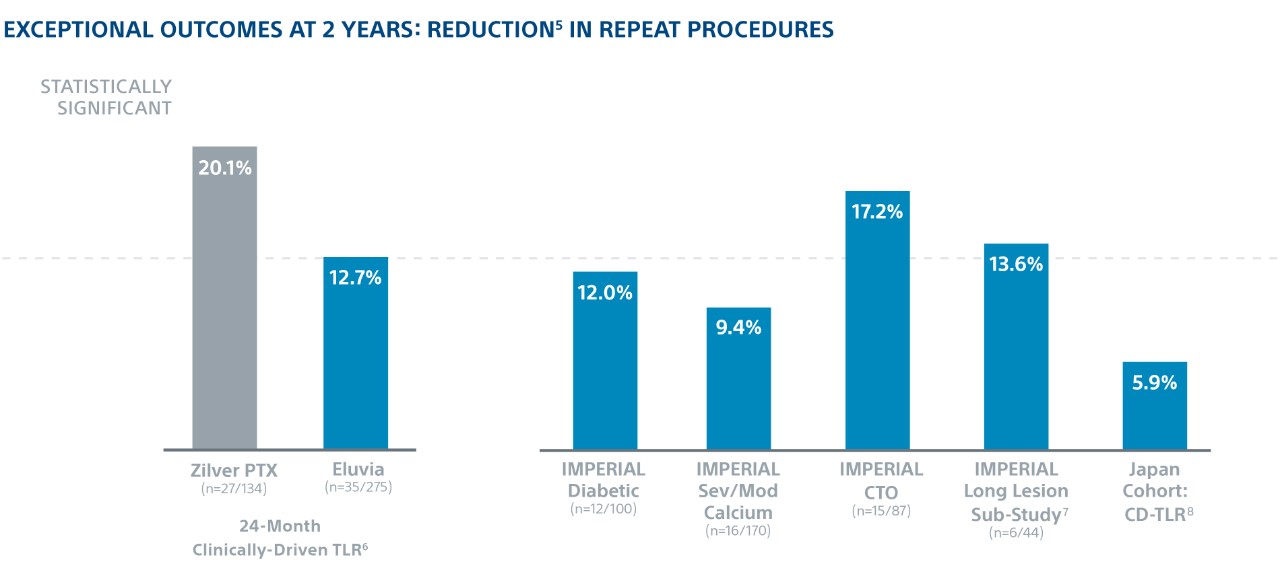
Exceptional Outcomes in Complex Lesions
In the world’s first head-to-head DES SFA trial, Eluvia demonstrated the highest ever reported 2-year Kaplan-Meier primary Patency of 83% and showed low 2-year TLR in patients with long lesions, diabetes, CTOs, and severe/moderate calcium.
As the only device using polymer to elute drug for PAD, Eluvia features the lowest drug dose with the fewest downstream particulates, leaving no paclitaxel in the bloodstream at 30 minutes post implant.
Eluvia Drug-Eluting Vascular Stent System UPN and GTIN Codes
| Description | UPN | GTIN |
| Eluvia 6 mm x 40 mm x 130 cm | H74939294600410 | 08714729876571 |
| Eluvia 6 mm x 60 mm x 130 cm | H74939294600610 | 08714729876588 |
| Eluvia 6 mm x 80 mm x 130 cm | H74939294600810 | 08714729876595 |
| Eluvia 6 mm x 100 mm x 130 cm | H74939294601010 | 08714729876601 |
| Eluvia 6 mm x 120 mm x 130 cm | H74939294601210 | 08714729876618 |
| Eluvia 7 mm x 40 mm x 130 cm | H74939294700410 | 08714729876694 |
| Eluvia 7 mm x 60 mm x 130 cm | H74939294700610 | 08714729876700 |
| Eluvia 7 mm x 80 mm x 130 cm | H74939294700810 | 08714729876717 |
| Eluvia 7 mm x 100 mm x 130 cm | H74939294701010 | 08714729876724 |
| Eluvia 7 mm x 120 mm x 130 cm | H74939294701210 | 08714729876731 |
| Product Code | - |
| Application | Drug-Eluting Vascular Stent System |
| Manufacturer Country | USA |
| Guarantee Type | Based on company recommendations |