Express SD Renal and Biliary Metallic Stent
Order Product Form

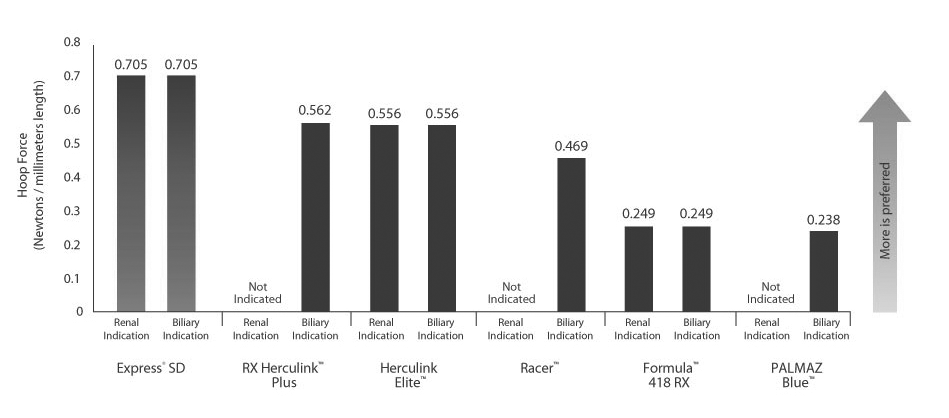
Best-in-Class Compression Resistance
Compression Resistance defined as the force required to compress the fully expanded stent. Compression Resistance measured using the hoop force tester in 37° C air.
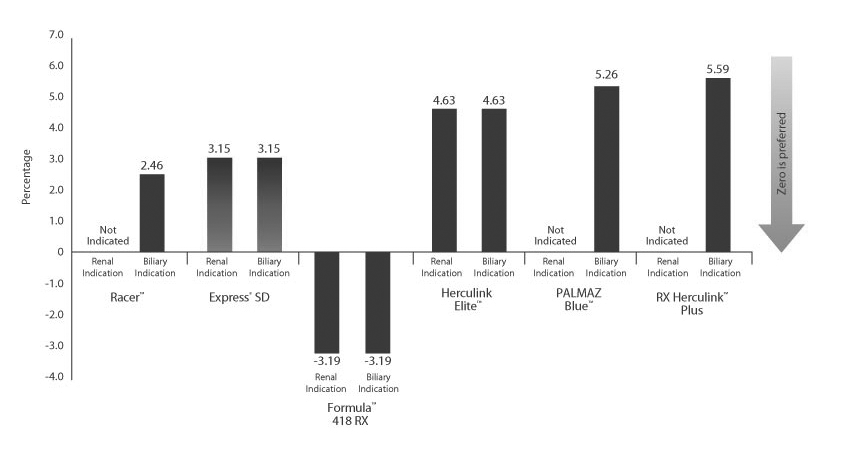
Minimal Foreshortening
Foreshortening defined as the change in stent length from crimped conditions to nominally expanded condition in 37° C water, as measured by a magnified vision system.
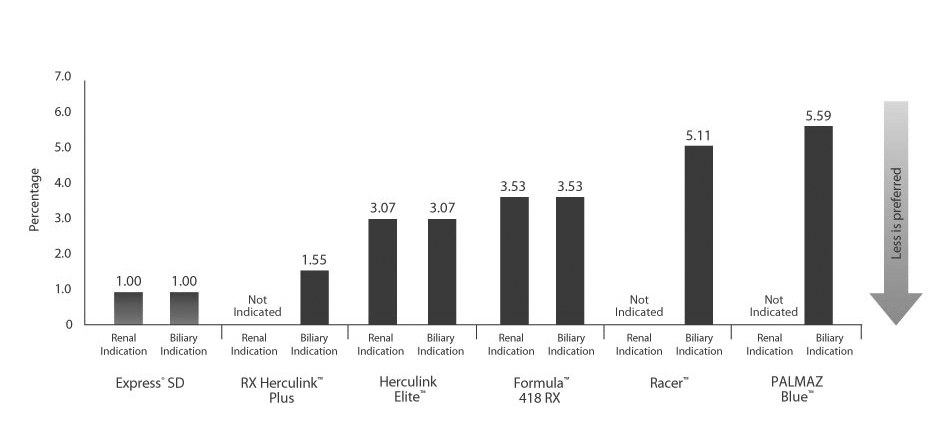
Very Low Recoil
Stent Recoil defined as the change in stent diameter from nominally pressurized catheter to non-pressurized catheter condition in 37° C water, as measured by a magnified vision system.
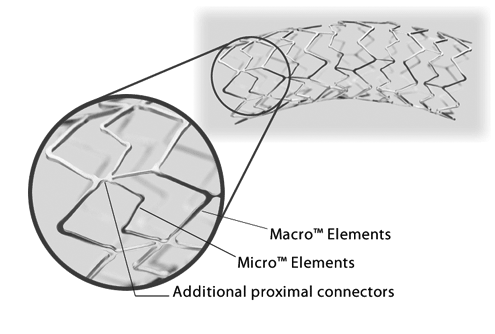
Express SD features a patented Tandem Architecture Stent Design
- Macro Elements and additional proximal connectors engineered to provide strength, especially in ostial lesions
- Micro Elements designed to provide flexibility during stent placement
Clinical Information
RENAISSANCE Clinical Trial
Overview The RENAISSANCE Trial was a prospective, multi-center, single arm study designed to evaluate the Express SD Renal Premounted Stent System in the treatment of atherosclerotic lesions in the aortorenal ostium.
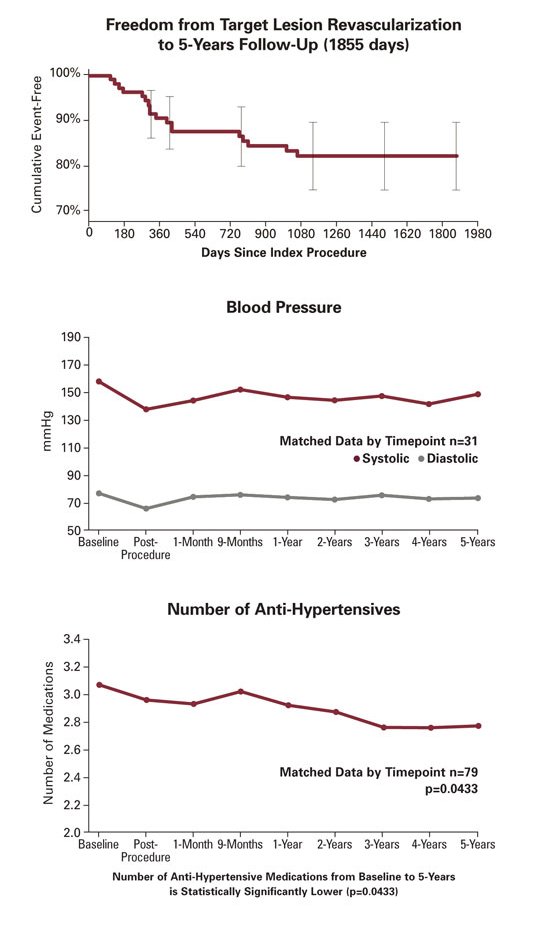
The primary objective was to demonstrate superiority of the duplex triggered, angiographically-confirmed binary restenosis rate at 9-months over the 40% pre-specified Objective Performance Criterion (OPC) representative of PTRA.
Trial Design The RENAISSANCE Trial enrolled 100 patients (117 lesions) with de novo or restenotic ostial atherosclerotic lesions ≤15mm long in vessels between 4.0 and 7.0mm diameter with diameter stenosis ≥70%.
Outcomes Primary Endpoint: 21.3% binary in-stent restenosis rate at 9-months, defined as the proportion of target lesions with ≥50% diameter stenosis based on angiographic core lab assessment.
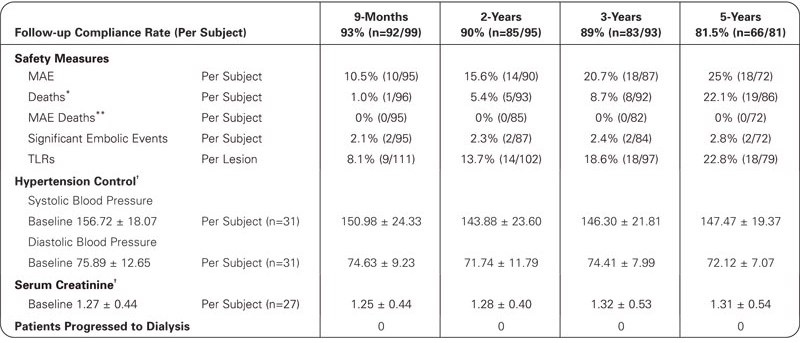
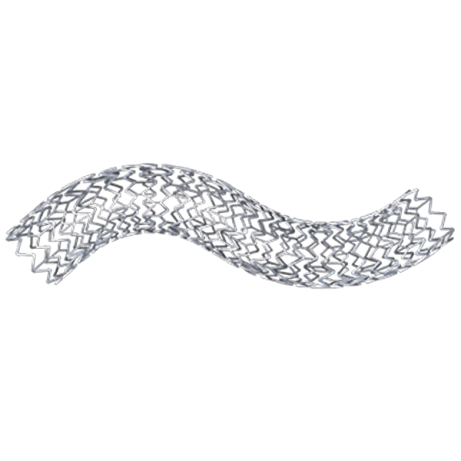
5-Year Data
Additional Results
*Denominator consists of number of subjects who either died prior to late follow-up window and/or had follow-up to at least the early follow-up window.
**Denominator consists of number of subjects who either had CEC adjudicated device or procedure related death prior to late follow-up window and/or had follow-up to at least the early follow-up window.
†Matched data by time point.
For additional information, please see Rocha-Singh K, Jaff M, Kelley E.L. Renal Artery Stenting with Non-Invasive Duplex Ultrasound Follow-up: 3-Year Results from the RENAISSANCE Renal Stent Trial. Catheterization and Cardiovascular Interventions 2008; 72:853-862.
| Product Code | - |
| Application | Renal and Biliary Premounted Stent System |
| Manufacturer Country | USA |
| Guarantee Type | Based on manufacturer recommendations |