- Home Page
- About
- Products
- News&Article
- Services
- Learning
- Contact
-
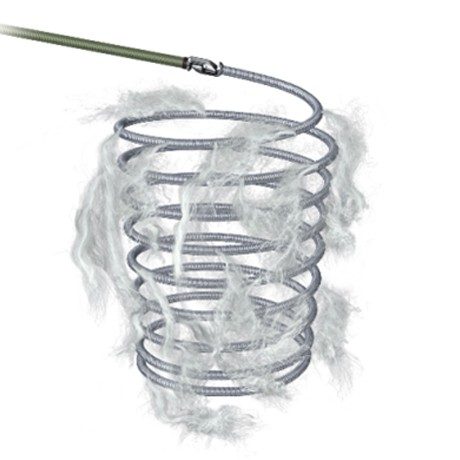
Interlock-35 Detachable Embolization Coils
Order Product Form



Shapes
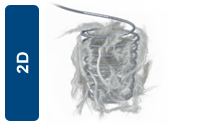 |
The 2D Helical shape is designed to provide optimal anchorability, as the helical shape allows the coil to be in constant, consistent contact with the wall of straight or tapered vessel. |
|---|---|
 |
The Diamond shape is designed to maximize occlusive power by tapering the distal and proximal ends of the coil, maximizing cross-sectional flow disruption. |
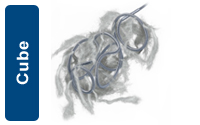 |
The Cube shape is designed to provide circumferential wall apposition and framing capability. The cube shape also provides cross-sectional filling, beneficial for use in aneurysms and packing large vessels. |
Fibered for Thrombogenicity Power
- Every coil contains multiple dense networks of synthetic fibers, each designed to catch blood cells and propagate the formation of thrombus.
- A close-up image of synthetic fibers (left) shows that the structure of the fiber strands are robust, yet porous, allowing for rapid thrombosis.
Catheter Compatibility
The Interlock-35 Coil is recommended for use with a 5F (1.70mm) diagnostic catheter - 0.035 in (0.89mm) or 0.038 in (0.97mm) inner lumen, such as Imager II Catheter without side flushing holes. Use of soft-wall catheter is not recommended due to significant resistance that could be encountered during advancement.
Physicians should exercise their clinical judgment in selection and use of catheters. Refer to the instructions for use for each product listed. Manufacturers may make changes to their catheters without notice which may impact their suitability for use with Interlock-35 Fibered IDC Occlusion System. Boston Scientific Corporation provides no warranty for use of third party catheters with its products. The use of other diagnostic catheters may result in an inability to deliver, deploy, or recapture the device.
Do not attempt to use the Interlock-35 Coil with a soft-walled delivery catheter, such as the Terumo Glidecath Catheter or the Angiodynamics SoftVu Catheter. Due to the coil’s high radial force and dense fibering configuration, significant resistance will be encountered when advancement through a soft-walled delivery catheter is attempted.
Coil Preparation


Nitinol pusherwire designed for control, strong pushability, and resistance to kinking when advancing and retracting the coil.
Ordering Information
2D Configurations
| UPN | Description | Diameter (mm) | Length (cm) | Shape |
|---|---|---|---|---|
| M001363500 | Interlock - 35 Coil | 3 | 4 | 2D |
| M001363520 | Interlock - 35 Coil | 4 | 10 | 2D |
| M001363540 | Interlock - 35 Coil | 6 | 10 | 2D |
| M001363550 | Interlock - 35 Coil | 6 | 20 | 2D |
| M001363570 | Interlock - 35 Coil | 8 | 10 | 2D |
| M001363580 | Interlock - 35 Coil | 8 | 20 | 2D |
| M001363590 | Interlock - 35 Coil | 8 | 40 | 2D |
| M001363600 | Interlock - 35 Coil | 10 | 20 | 2D |
| M001363610 | Interlock - 35 Coil | 10 | 40 | 2D |
| M001363620 | Interlock - 35 Coil | 12 | 20 | 2D |
| M001363630 | Interlock - 35 Coil | 12 | 40 | 2D |
| M001363640 | Interlock - 35 Coil | 15 | 20 | 2D |
| M001363650 | Interlock - 35 Coil | 15 | 40 | 2D |
| M001363660 | Interlock - 35 Coil | 18 | 20 | 2D |
| M001363670 | Interlock - 35 Coil | 18 | 40 | 2D |
Cube Configurations
| UPN | Description | Diameter (mm) | Length (cm) | Shape |
|---|---|---|---|---|
| M001363700 | Interlock - 35 Coil | 4 | 6 | Cube |
| M001363720 | Interlock - 35 Coil | 6 | 10 | Cube |
| M001363730 | Interlock - 35 Coil | 6 | 20 | Cube |
| M001363760 | Interlock - 35 Coil | 8 | 20 | Cube |
| M001363790 | Interlock - 35 Coil | 10 | 25 | Cube |
| M001363800 | Interlock - 35 Coil | 10 | 40 | Cube |
| M001363810 | Interlock - 35 Coil | 15 | 25 | Cube |
| M001363820 | Interlock - 35 Coil | 15 | 40 | Cube |
| M001363830 | Interlock - 35 Coil | 20 | 40 | Cube |
Diamond Configurations
|
UPN |
Description |
Diameter (mm) |
Length (cm) |
Shape |
|---|---|---|---|---|
|
M001363910 |
Interlock - 35 Coil |
4 |
4.5 |
Diamond |
|
M001363920 |
Interlock - 35 Coil |
6 |
9 |
Diamond |
|
M001363930 |
Interlock - 35 Coil |
8 |
14 |
Diamond |
| Product Code | - |
| Application | Embolization Coils |
| Manufacturer Country | USA |
| Guarantee Type | Based on manufacturer recommendations |











